Project description
CHARGE Syndrome is a rare developmental disorder which affects 1/ 10 000 newborns and has a very complex clinical presentation. Heterozygous mutation of CHD7 (Chromodomain helicase DNA-binding domain) is currently the main genetic cause of CHARGE Syndrome. This condition belongs to the neurocristopathies family, which are diseases of neural crest cell development, but underlying molecular mechanisms are not well understood.
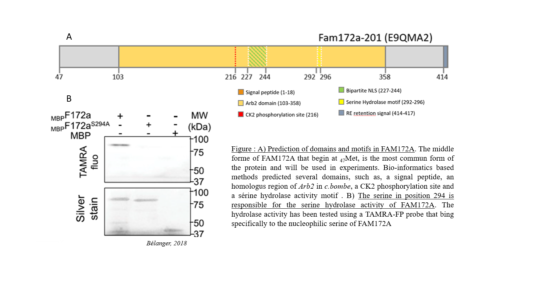
The Pilon lab recently identified FAM172A as a new candidate gene for CHARGE Syndrome. Preliminary studies revealed that FAM172A could regulate the co-transcriptional alternative splicing machinery, through interaction with Argonaute 2 (AGO2), DNA and RNA. We hypothesize that dysregulation of alternative splicing, along with transcriptional landscape perturbation specific to neural crest cells are the cause of CHARGE Syndrome. Bioinformatics-based analyses further predicted several functional domains in FAM172A, such as a consensus motif for Casein Kinase II (CK2) phosphorylation, and an esterase-like serine hydrolase activity. In order to better understand the molecular mechanism of FAM172A and its role in co-transcriptional alternative splicing events, as well as its implication in CHARGE Syndrome, these two motifs will be carefully studied during my PhD project.
Research team
Name: Séphora Sallis, M.Sc
Supervisor: Nicolas Pilon (UQAM)
Laureate: Doctoral fellowship 2019